Chronic heart failure patients draw hope from a new technology. A team of life science entrepreneurs in Houston, Texas has developed the first catheter-deployed circulatory assist device intended for long-term use. Procyrion, Inc.’s Aortix™ provides a minimally invasive treatment option for the more than two million chronic heart failure patients in the USA alone who are too sick for medication. This pre-clinical cardiologist tool dramatically reduces risks associated with circulatory support devices and enables treatment of younger, healthier patients before progressive damage occurs.

The brushless DC motor EC 6 is the second smallest drive in the maxon family. The compact form makes it especially suitable for medical applications.
Assisting the natural function of the heart, the intra-aortic pump has been thoughtfully designed as an alternative to large, cumbersome surgical devices currently providing full circulatory support. Unlike these devices, Aortix provides minimal procedural risk. Measuring approximately 6 mm in diameter and 6.5 cm long, a cardiologist can deliver Aortix via a catheter in the femoral artery to the descending thoracic aorta. Once the catheter sheath is retracted, the self-expanding nickel-titanium anchors deploy to affix the pump to the aortic wall. Aortix accelerates a portion of the body’s native blood flow within the pump and pushes it through fluid entrainment ports directed downstream. The jets entrain native aortic flow, transferring energy to the cardiovascular system and increasing blood flow to vital organs such as the kidneys. Additionally, in a model of chronic heart failure, Aortix decreased energy consumption of the heart by 39 percent, allowing the heart to operate more efficiently, encouraging cardiac rehabilitation and recovery.
Procyrion has been working with maxon for almost two years to develop a motor for this unique and demanding application. The basis for the Aortix device is a maxon EC6 motor with some customization including the electrical lead, shaft length, and bearing assemblies - all designed to make the pump durable and biocompatible. maxon also designed a high efficiency motor core for this application, which extends battery life and produces less heat so it doesn’t adversely affect the circulating blood. In addition, maxon is working closely with Procyrion to implement a magnetic torque drive, so the motor could be mounted inside a hermetically sealed chamber. This configuration eliminates the possibility of blood entering the motor core. The magnetically coupled pump arrangement is a method sometimes used for giant pumps in the oil field, but because of maxon’s breadth of experience across multiple industries, the company was able to help the Procyrion team successfully transfer this technology to a miniature scale medical application
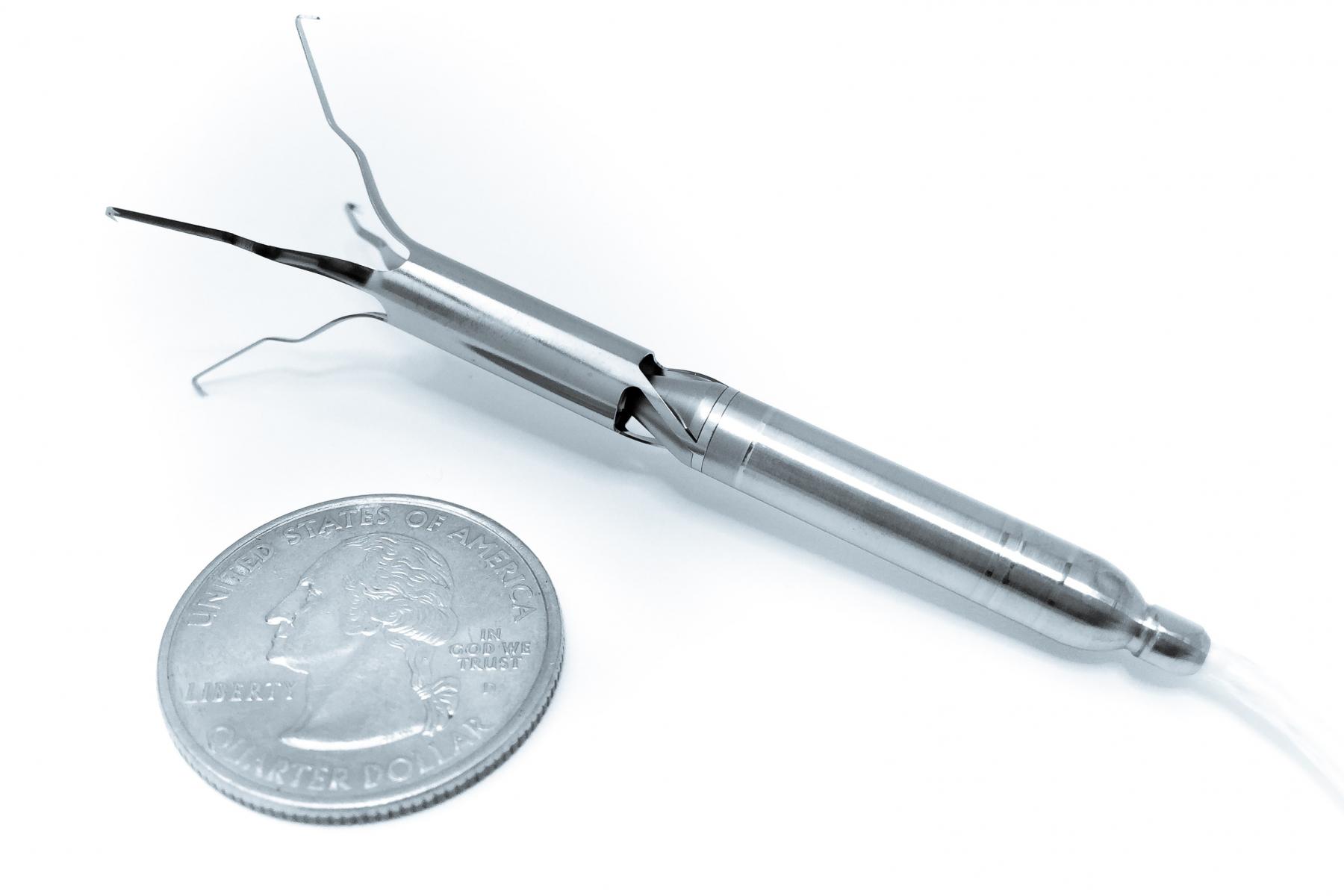
Anchors affix the pump to the aortic wall.
Each Aortix device consists of a small, continuous flow pump mounted within a selfexpanding anchoring system. The anchored pump attaches to a flexible power lead, which can be tunneled to a desired transdermal exit site or to a Transcutaneous Energy Transfer (TET) system for subcutaneous implantation without an indwelling power lead. Presently, the device can operate for over eight hours on a single battery pack. The external battery pack and control unit have been designed to be “hot swappable”, meaning the battery can be changed without needing to stop the device. A variety of charging devices can be used.
The Procyrion team has also built a TET charging system that enables the battery to be charged wirelessly. This design has the potential to significantly reduce the risk of infection, common with other implantable heart pumps. Because traditional assist devices replace heart function rather than support it, device failure can be fatal. With Aortix, a partial support device which doesn’t obstruct native blood flow, failure is not life threatening. Should the pump fail, the device can easily be retrieved and replaced in another minimally invasive, catheter-based procedure.